This blog has been archived. A new one has been set up at this link.

Andreas Mortensen, vice president for research at Swiss federal technology university EPFL, said the COVID-19 crisis has made international R&D collaboration stronger than ever. But some actors will need more help than others, and startups will be probably hard hit if they can’t promote their ideas in different countries. At the same Science|Business online event 22 April, Robert-Jan Smits, president of Eindhoven University of Technology, also noted that there will be changes to global supply chain and global partnerships, which will certainly have an impact on research.
Robert-Jan Smits also added that it won’t be the strongest actors in the research ecosystem that will survive, but rather the most adaptive ones. Governments should provide support for example by giving financial aid while asking innovation acceleration such as energy transition, in line with the EU Commission's Green Deal ambitions.
President Trump may be taking an “America First” approach to COVID-19 remedies, but European Commission officials are trying to stress a more globalist view of what’s needed to end the crisis.
Jean-Eric Paquet, the EU’s director general for research and innovation, told an online audience 20 April that the “hundreds of millions of euros” that the Commission has committed to COVID-19 research so far is for solutions “that need to be available globally. This is the value proposal: Vaccines for the world, therapies for the world. This is about finding medicines and the cures, but also finding ways to upscale them very fast and distribute them universally as far as possible. That’s what Europe’s solidarity proposition is about….We want to find solutions for the world.”
His comments echo other senior Commission officials in the past few weeks – but in truth, solidarity has become a vexed political issue inside as well as outside the EU. Inside, as EU members debate COVID-19 rescue packages, many southern European politicians are accusing northerners of being stingy and letting the COVID-stricken south suffer unaided. And EU relations with Washington were upended last month when the Trump Administration reportedly tried to get control of a German vaccine company’s output; it led in short order to the EU granting the company an €80 million financing package to keep its work open.
Paquet was speaking at a promotional video conference for an EU “Hackathon”, from 24-26 April, a bit of online political theatre that is expected to gather more than 60,000 participants to brainstorm possible solutions – technical or otherwise - for the COVID-19 crisis. “You don’t need to be a scientist” to join the session, Paquet said.
The Israeli Ministry of Defense is backing a project by the Afeka Tel-Aviv College to detect pre-symptomatic COVID-19 carriers using voice and speech processing. The study will develop a mobile app to identify potential carriers of the virus, to help break the chain of infection. Speech samples are being collected from volunteers by the startup Vocalis Health, which launched the ‘voice prints’ project in March.
The European Patent Office announced the 2020 European Inventor Awards, due to be held in Monaco on 17-18 June, have been postponed. The event will take now place in Monaco on 17 June 2021.
German biotech BioNTech, which is working with Pfizer to develop a COVID-19 vaccine, announced today the German regulator, the Paul-Ehrlich-Institut, has approved the Phase I/II clinical trial of its BNT162 vaccine, which will be the first clinical study of a COVID-19 vaccine candidate in Germany. The company has designed four vaccine candidates that deliver the genetic code for four different COVID-19 antigens. The dose escalation portion of the Phase I/II trial will include approximately 200 healthy subjects between the ages of 18 to 55, to determine the optimal dose for further studies, as well as evaluating safety and immunogenicity. The study will also evaluate the effects of repeated immunisation for three of the four vaccine candidates. Pfizer is due to start a US trial as soon as it gets regulatory approval. BioNTech will supply the vaccine candidates from its GMP-certified mRNA manufacturing facilities in Europe.
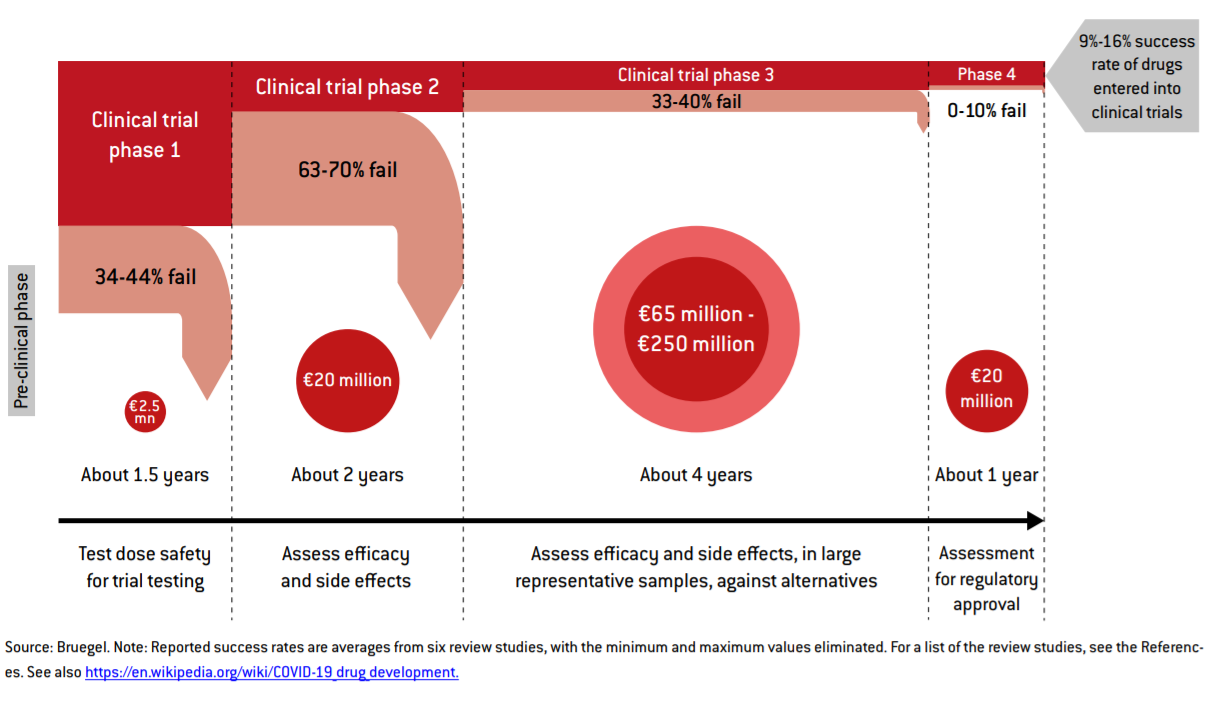
A new Bruegel report published today highlights the need for more investment in the later stages of clinical development of COVID-19 vaccines and says more should be done to support bioprocess development and scale up of manufacturing, at the same time as vaccines are being tested, rather than waiting until trials show it is clear they are effective.
The report, co-authored by Reinhilde Veugelers, a former member of the European Research Council’s scientific council and adviser to R&D commissioner Maire Geoghegan-Quinn calls for more public funding to reduce the risk for companies running vaccine projects. A staged support scheme, with a certain number of grants of a certain size, for each of the three phases of clinical development, will ensure at least three vaccines emerge from the end of the pipeline, ready for regulatory approval. The total budget would range from €725 million to €3 billion, depending on how many projects drop out.
Currently, five COVID-19 vaccines are in phase I human trials in the US and China. A vaccine developed at Oxford University will be the first to enter the clinic in Europe, with the first human volunteers due to receive the vaccine tomorrow (23 April).
The venture capital arm of the French insurance company Axa, is putting €5 million into COVID-19 research to inform responses in the aftermath, as well preparing for a potential second wave, particularly in lower income countries, and looking into how to better prepare for the next crisis, of whatever cause. The call for proposals is directed at mid-career PhDs working in academic institutions. Grants of up to €250,000 are on offer. Application deadline: 7 May.
The Spanish government announced two measures to shield research and development from COVID-19, saying it will extend the contracts of PhD students for the duration of the crisis and that it will postpone repayments on state loans to science and technology parks. Repayments due in 2020 will be transferred to the same date in 2021.
Recent seizures of fake medical supplies being marketed as protection against Covid-19 have underscored the need to deal with the growing international trade in counterfeit pharmaceuticals, according to the OECD and the EU Intellectual Property Office, which today publish a joint report, ‘Trade in Counterfeit Pharmaceutical Products’, and an accompanying brief on links with the COVID-19 crisis. “The discovery of fake medical supplies related to COVID-19, just as the world pulls together to fight this pandemic, makes this global challenge all the more acute and urgent,” said OECD secretary general Angel Gurría. “We hope the evidence we have gathered on the value, scope and trends of this illicit trade will help lead to rapid solutions to combat this scourge.”
A €7.2 million Horizon 2020 funded project which is using high precision plant breeding techniques to modify tobacco plants so that they produce substances other than nicotine, is making the genome sequence of an Australian tobacco plant, Nicotiana benthamiana publicly available. Genetically modified tobacco plants are used to produce proteins for use in biological drugs, for example, N. bethamiana has been used to generate antigens for use in flu vaccines. The researchers at the Newcotiana project say their high quality genome sequence, now available to public and private researchers, will help in understanding and optimising the genes that control the quantity and quality of biopharmaceutical compounds produced in the plant. The Newcotiana project, coordinated by the Spanish National Research Council and led by Technological University of Queensland, also involves 13 other EU research institutions.

 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.