A drop off in the number of commercially led trials has had an opportunity cost for UK taxpayers of an estimated £360 million over the past five years, says government commissioned review

The UK government cannot let attempts to revitalise its commercial clinical research activity fall down its list of priorities or it will lose further ground on competitors and miss out on huge financial gains for its healthcare service.
The warning comes from James O'Shaughnessy, member of the House of Lords and former parliamentary under-secretary in the department of health, who recently authored an independent report on the matter and spoke in front of the House of Lords Science and Technology Committee today.
The UK remains extremely strong in recruiting patients to participate in academic trials, but in recent years, particularly since the COVID-19 pandemic, its status as a world leader in commercial clinical trials has been dropping rapidly.
Commercial clinical trials are funded by private companies such as those in the pharmaceutical or medical devices industry and play a crucial role in the development of new treatments. In the period between 2017 to 2018 and 2021 to 2022, the number of patients enrolled onto commercially led studies in the UK fell from around 50,000 per year to 28,000, figures from the Association of the British Pharmaceutical Industry show.
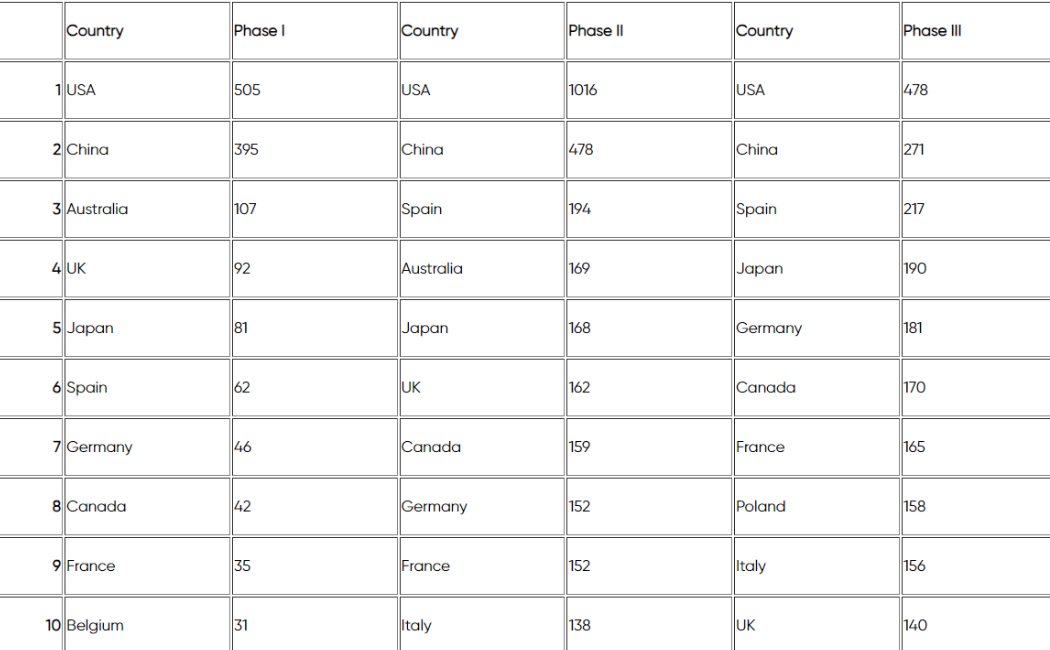
The UK has also dropped from fourth to 10th in the world in terms of the number of phase III industry trials it carries out, although it is still in fourth position for phase I trials, which are more likely to be funded with public money.
A major reason that the UK should prioritise commercial clinical trials is the huge financial burden it can remove from the public purse. The total direct cost of the number of patients involved in these trials nearly halving in the past five years is around £360 million, O'Shaughnessy states in his report. “Over this time period, an additional estimated £570 million could have been provided to the NHS to recover costs of running commercial trials,” he states.
Part of the issue, according to O'Shaughnessy, is that the UK channelled so much money and effort into Covid-related research that “everything else stopped”.
“More than one person in this review told me that ‘if only we could just go back to where we were five years ago’…,” he told the House of Lords committee. “We were doing better five years ago so you can understand the impulse. But the world has changed. We know how to do radically better. We can do not just as well as we were doing five years ago, but twice as well.”
This feeling is reflected in his review, in which he proposes that the government should aim to double the number of people taking part in commercial clinical trials in the next two years, and then double it again by 2027.
Added to the impact of the pandemic is the fact that the UK is, compared to its global competitors, increasingly slow at setting up commercial clinical trials and recruiting patients to them.
This is in no small part down to reduced resources and capabilities of the Medicines and Healthcare products Regulatory Agency, O'Shaughnessy said.
Another issue is the tracking and analysis of clinical trials in the UK, with ones that perform well given no more incentives or funding than ones that perform poorly. On top of that, investment into R&D is little more than a footnote in the NHS Long Term Plan.
“It is just not a priority for the NHS leadership,” O'Shaughnessy said. “It’s two-and-a-half pages in the Long Term Plan. There is no R&D target for the NHS, even though it’s meant to be one of our key industries for the long-term future of the UK.”
UK responds to report
The UK government last month announced it was investing £121 million into tackling five areas highlighted as issues in O'Shaughnessy’s report, including improving access to clinical trial data and speeding up contracting processes to reduce delays when studies are carried out at multiple sites.
The report included 27 recommendations, but O'Shaughnessy said that the five areas the UK has initially targeted are the more important ones. “There was nothing I asked for in that regard that didn’t come through,” he said. “I’m happy with the funding allocated.”
The government has also committed to developing an action plan on how it intends to implement the 27 recommendations by this autumn, O'Shaughnessy said.
But his biggest concern is that this new focus on improving commercial clinical trial activity falls down the priority list, especially with a general election on the cards for 2024.
“They’ve put this initial tranche of money towards it but…it is all about execution risk,” he said. “The biggest risk is that among everything else, it is a second-order priority, which I don’t think it should be.”
Another vital element is changing the mindset around the role of clinical trials in society.
“Almost every country in the world has tremendous pressure on its healthcare services, and yet others are managing to leap ahead in this area: Spain, Australia, Poland…” O'Shaughnessy said. “So, it clearly is possible, but it is only possible if it matters to somebody who has the power to do something about it…but culturally we’re not there yet.”





 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.