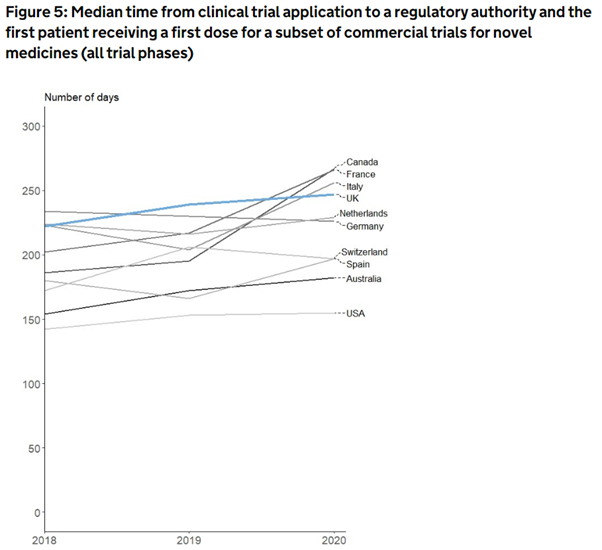
 The United States remains the quickest country to get new medicines from clinical trial applications into patients, according to new data from the UK.
The United States remains the quickest country to get new medicines from clinical trial applications into patients, according to new data from the UK.
On average, it took a 155-day turnaround time in the US to approve trials and then set up a clinical study, including recruiting patients. In the UK, it look nearly 100 days longer on average.
Across all countries compared, the process of setting up clinical trials has been getting slower since 2018, found the analysis, Life science competitiveness indicators 2022, an annual report released by the government.
However, the times taken in 2020 were likely impacted by the Covid-19 pandemic, when countries scrambled to switch their research efforts to the novel virus, the analysis says.
Prior to the pandemic in 2019, the UK was the slowest among comparator countries at getting clinical trials up and running.





 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.