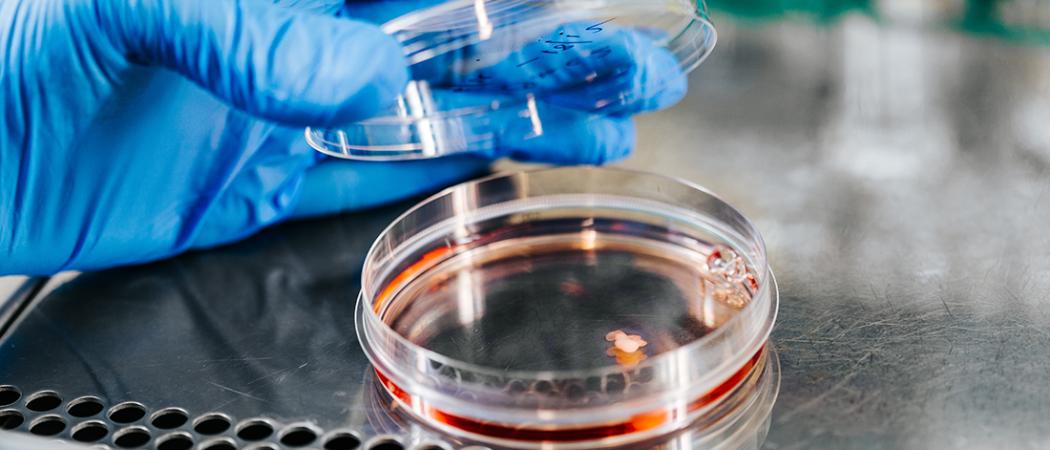
It’s dangerous and complicated to extract brain cells from humans. Instead, geneticists prefer to reprogramme skin cells to become brain cells. The results may eventually lead to cures for various diseases. Photo: Julie Gloppe Solem, NTNU
By reprogramming skin cells to become brain cells, researchers have managed to cultivate lots of mini human brains. Some of them have begun to grow pupils for eyes. The technique helps researchers study the most minute details of the genetics of turning stem cells into other cells.
“Would you like to see the mini-brains we’ve created?” asks Magnar Bjørås, Professor of Medicine at NTNU. “We have lots of mini-brains in the lab. We made them from skin cells,” he adds.
How else can a reporter respond but, “Yes. Sure… of course. But wait a minute, are they real?”
“We recoded skin cells to become brain cells. Then we let them sit and grow,” Bjørås responds matter of factly.
Black dots are small pupils
It’s as if reprogramming cells is as common as eating a slice of bread for lunch – and for some researchers it may be. From a kind of fridge Bjørås extracts a glass of something that resembles diluted red juice.
And inside, like little snowflakes, the mini-brains are swirling around. It’s hard to believe, but these cells actually live in a regular lab on the third floor at NTNU in Trondheim. In fact, the fridge is home to lots of red juice glasses containing mini-brains.
I notice black dots on the mini-brains and ask what they are.
“It looks like pupils are growing for eyes. If we supplied blood to the mini-brains, they would grow into pupils and everything. It’s possible to do, but really complicated. So we haven’t done that yet,” says Bjørås.

“The ageing population is an important patient group,” says Bjørås. “When you get to be about 70 years old, you run out of neural stem cells. With gene therapy you can increase the number of these cells. You can also cure Parkinson’s disease, which occurs when a particular type of cell dies.” Photo: Julie Gloppe Solem, NTNU
He hasn’t done that. Yet. Hmm, right.
- You may also like: Norwegian family’s medical mystery solved
Creating a mouse from a skin cell
“A lot is going to be happening in this field in the future. Humans have already undergone gene therapy. The technology to modify the approximately 20 000 ‘instruction books’ in the codebook for the human genetic code already exists,” says Bjørås.
All of this is clearly completely commonplace for a man who since childhood has been extremely curious about understanding the invisible puzzle that constitutes all living beings.
Indeed, growing new brain cells, creating new organs and changing an individual’s instruction book may all become commonplace sometime in the future.
A lot has certainly happened since Tiny the mouse was born eleven years ago after being created from genetically reprogrammed skin cells. Maybe the future where all of this is commonplace is right now. Changes are happening at a furiously fast pace.
Lamb incubated in artificial womb
Last year, the world’s first genetically modified salmon came onto the market at stores in Canada. Its genes were altered so that they could not reproduce with wild salmon. A premature lamb was also successfully brought to term in an artificial womb last year. The CRISPR genome editing method has been tested on both humans and embryos. CRISPR technology uses an enzyme to bind to parts of an organism’s DNA that cause disease – or whatever else one wants to remove – and cuts it. This shuts off the targeted gene so that it is gone forever. The method can cut out hereditary cancer, HIV or Alzheimer’s disease.
“Our research group cooperates closely with the Kavli Institute at NTNU. To put it simply: they find the cells and we study what’s inside the cells. What I’m trying to understand is the genetics – down to the atomic level of the cells,” Bjørås says.
- You may also like: Using Big Data to understand immune system responses
Creating new organs
Bjørås plans to use this knowledge to identify what keeps a cell healthy, what causes illness and how to create new organs.
“It’s dangerous and complicated to extract brain cells from humans. That’s why we reprogramme skin cells to become brain cells instead. You can also use stem cells from embryos. They can be used to become anything: brain cells, skin cells or liver cells,” says the researcher.
“What we’re doing so far is basic research,” he explains, “but with gene therapy you can introduce genetic material, like brain cells, into a sick patient. If the disease is due to a gene failure that causes a particular gene not to produce functional protein, the genetic defect can be corrected so that the cell produces healthy protein.”
“You can also transplant genes that give the cells disease-fighting properties. Another example is genes that enable immune cells to recognize and kill cells that cause disease,” says Bjørås.
- You may also like: Hoping to treat osteoarthritis using cartilage tissue
Isolated from the world
Gene therapy is nothing new, really, although tools like CRISPR are new. Gene therapy was first tried in 1990 when four-year-old Ashanti DeSilva received treatment for the hereditary genetic disease ADA-SCID in the United States. The disease is life-threatening because the immune system is unable to fight off bacteria, viruses or fungi.
Left untreated, very few children live longer than two years. DeSilva quickly recovered after her treatment and no longer needed to be isolated from the outside world. This opened the door for a number of gene therapy trials on patients with various hereditary diseases in subsequent years, and many of them became healthy.
Gene therapy at the time was a challenging landscape that was technically difficult, and some patients suffered serious side effects from treatment. In two cases, patients died as a result of side effects, leading to a slowdown of developments in the field. But for many people with life-threatening illnesses, gene therapy has been their only hope. Gene therapy methods have gradually improved and decreased the risk of serious side effects significantly.
The CRISPR method can remove cancer or other inherited diseases – forever. Illustration photo: Thinkstock
Many types of gene therapy are being tested for a variety of diseases today, and they are expected to be of great importance to medical treatment in the future. In 2016, gene therapy for ADA-SCID was approved in the EU – almost 30 years after the first trials took place.
Giving older people new brain cells
According to Bjørås, the ageing population is an important potential patient group. “When you reach the age of 70 or so, you run out of neural stem cells. With gene therapy you can increase the number of these cells. You can also cure Parkinson’s disease, which occurs when a particular type of cell dies,” he says.
It may take some time before gene therapy methods become widely available to patients, since the time from the research stage until a treatment is approved usually takes several years. Some of the possibilities of gene therapy have been approved, but most gene therapies will probably also be very expensive. The Norwegian Biotechnology Advisory Board states on its website that offerings for treatment will largely become a matter of prioritizing health care resources.
Links to articles by Magnar Bjørås:
Synergistic Actions of Ogg1 and Mutyh DNA Glycosylases Modulate Anxiety-like Behavior in Mice. https://www.ncbi.nlm.nih.gov/pubmed/26711335
NEIL3-Dependent Regulation of Cardiac Fibroblast Proliferation Prevents Myocardial Rupture. https://www.ncbi.nlm.nih.gov/pubmed/28052262





 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.