This blog has been archived. A new one has been set up at this link.

The coronavirus pandemic is disrupting universities and research institutes across the world. But the same institutions are also working very hard to find out how the disease can be stopped and its effects mitigated.
Follow this live blog for the latest updates on how the crisis is impacting research and innovation, and what governments, funders, companies, universities, associations and scientists are doing to stop or cope with the pandemic.
You can read the full archive of this blog here.
The European Medicines Agency (EMA) has concluded there is a possible link between Johnson & Johnson’s single dose COVID-19 vaccine and eight cases of unusual blood clots seen in people who received the vaccine in the US, but said the benefits of vaccination outweigh the risks.
A warning about the rare clots will be added to the label and listed as a very rare side effect of the vaccine.
EMA looked at all the currently available evidence, which consisted of eight reports of the clots occurring among over seven million people who had received Johnson & Johnson’s vaccine in the US, as of 13 April.
Following the reports, use of the vaccine was put on hold in the US and Johnson & Johnson said it would be delaying rollout in Europe, telling member state governments to put supplies into storage.
Following a meeting on 14 April the US Food and Drug Administration and the Centers for Disease Control, jointly decided they needed more time to deliberate and extended the hold. They are expected to announce a decision on Friday (23 April).
EMA said it was able to conclude its review sooner because of its experience in looking at similar rare clots that have been seen in people who received the AstraZeneca vaccine.
It is possible to treat the rare clots, EMA said. Now the warning is on the label and healthcare providers and the public have been alerted to the symptoms, it will be up to member states to decide on the rollout of the Johnson & Johnson vaccine. That decision should be based on the level of infection, the number of people in hospital and intensive care, and the availability of other vaccines, EMA said.
“EMA’s scientific assessment underpins the safe and effective use of COVID-19 vaccines. Use of the vaccine during vaccination campaigns at national level will take into account the pandemic situation and vaccine availability in individual member states,” the agency said.
The UK government has set up a pandemic preparedness partnership to advise the G7 group of nations on how to harness international collaboration to intensify research and development, modernise clinical trials and improve vaccines manufacturing and supply chains.
The partnership, meeting formally for the first time today, will be backed by £16 million in additional funding from the UK government to support the work of the Coalition for Epidemic Preparedness Innovations (CEPI) on global vaccine supply.
The public private partnership, chaired by UK government chief scientific adviser Patrick Vallance, brings together industry, international organisations and leading experts. It will provide recommendations for delivering on targets to more quickly develop vaccines, therapeutics and diagnostics, through greater global cooperation on research and development, manufacturing, clinical trials and data sharing.
The partnership will report to leaders at the G7 summit, to be held in Cornwall in June, which the UK is hosting as current president of the G7.
The £16 million investment in CEPI will fund global vaccine manufacturing capacity and R&D to rapidly respond to the threat of new variants of SARS-Cov-2, supporting the development of variant-specific vaccines.
The aim is to have millions of doses of a vaccine available for emergency use by 100 days after a variant of concern is identified.
“COVID-19 has shown us that it’s possible to develop and deploy high quality vaccines much faster than previously imagined,” Vallance said. “We have brought together the pandemic preparedness partnership to see whether this can be accelerated even further and applied to the development of medicines and diagnostic tests.
SARS-CoV-2, the virus that causes COVID-19, can mutate in mink in a way that reduces immune control by antibodies, according to new research carried out at the German Primate Centre at the Leibniz Institute for Primate Research in Göttingen.
The research shows that an antibody used in an approved COVID-19 therapy is unable to effectively inhibit SARS-CoV-2 harbouring a spike mutation acquired in mink and that the mutation also reduces the effect of neutralising antibodies produced in people following infection with SARS-CoV-2.
It has been known for about a year that mink can become infected with SARS-CoV-2 and that the virus had been transmitted from humans to farmed mink and has mutated in infected animals.
Mutations were acquired in the spike protein, via which the virus enters human cells, with these SARS-CoV-2 variants then being transmitted back to humans, raising concerns that mink could be a continuing source of infection of humans.
As a result, in June 2020 the Danish government ordered a mass cull of 15 million farmed mink to prevent the transmission of new viral variants to humans. Other European governments have also ordered control measures.
Researchers at the German Primate Centre looked at a number of mutations found in the spike protein in farmed mink, including Y453F. “Our results show that one of two antibodies from an antibody cocktail used for COVID-19 therapy no longer efficiently inhibits the viral variant with the Y453F mutation,” said researcher Markus Hoffman.
“Furthermore, our study demonstrates that the Y453F mutation reduces inhibition of the virus by antibodies produced by COVID-19 patients. This means that people who were infected with SARS-CoV-2 may have reduced protection against mink variants of the virus", Hoffman said.
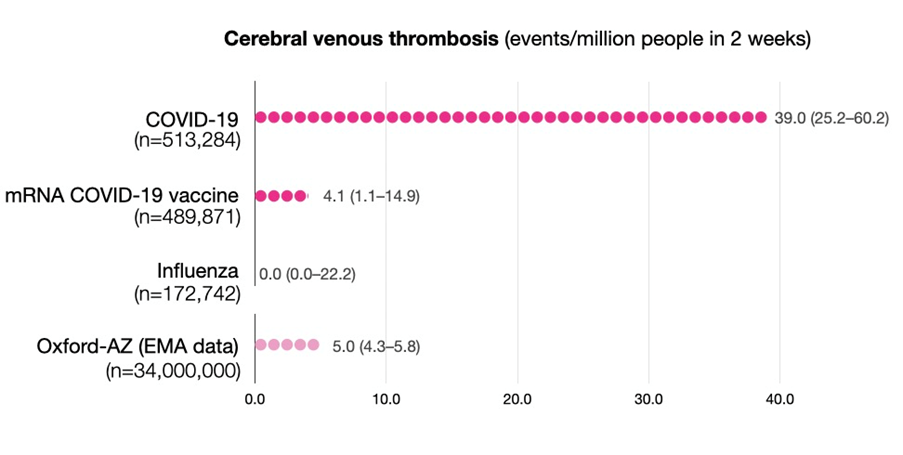
Researchers at Oxford University have reported the risk of rare blood clots (cerebral venous thrombosis, CVT) following COVID-19 infection is around 100 times greater than normal and several times higher than the risk after vaccination, or following a bout of influenza.
Paul Harrison, professor of psychiatry at Oxford University, who led the research, said, “There are concerns about possible associations between vaccines and CVT, causing governments and regulators to restrict the use of certain vaccines. Yet, one key question remained unknown: ‘What is the risk of CVT following a diagnosis of COVID-19?’”
“We’ve reached two important conclusions. Firstly, COVID-19 markedly increases the risk of CVT, adding to the list of blood clotting problems this infection causes. Secondly, the COVID-19 risk is higher than seen with the current vaccines, even for those under 30; something that should be taken into account when considering the balances between risks and benefits for vaccination,” Harrison said.
The researchers counted CVT cases diagnosed in the two weeks following diagnosis of COVID-19, or after the first dose of a COVID-19 vaccine. They then compared these to the incidence of CVT following influenza, and to the background level in the general population.
They found CVT is more common after COVID-19 than in any of the comparison groups, and that 30% of these cases occurred in people under 30 years of age. The risk compared to current COVID-19 vaccines is between 8-10 times higher, and compared to the normal background level, approximately 100 times higher.
- In the study of over 500,000 COVID-19 patients, CVT occurred in 39 in one million patients.
- In over 480,000 people who received a COVID-19 mRNA vaccine (Pfizer or Moderna), CVT occurred in 4 in one million.
- CVT has been reported to occur in about 5 in one million people after first dose of the AstraZeneca COVID-19 vaccine.
- Compared to the mRNA vaccines, the risk of a CVT from COVID-19 is about 10 times greater.
- Compared to the AstraZeneca, the risk of a CVT from COVID-19 is about 8 times greater.
However, all comparisons must be interpreted cautiously since data are still accruing.
Negotiations have begun on a third advance purchase agreement for 1.8 billion doses of the Pfizer/BioNTech COVID-19 vaccine, with the European Commission stipulating that manufacturing of the product and all the components that go into it must be based in the EU.
The doses will be delivered from 2021 to 2023. Commission president Ursula von der Leyen announced the negotiations as the EU reached the milestone of administering 100 million vaccinations. Of these, more than a quarter were second doses.
To date, member states have received a total of 126 million doses of vaccine, and after a stuttering start, vaccination rates are picking up. But another delay is looming, with rollout of Johnson & Johnson’s vaccine now on hold pending investigation into unusual blood clots in six people who received the product in the US.
This comes on top of holds in a number of member states on using AstraZeneca’s vaccine, also put in place as a result of concerns about of blood clots that the European Medicines Agency said is a rare side effect.
“As we can see with the announcement by Johnson & Johnson yesterday, there are still many factors that can disrupt the planned delivery schedule of vaccines,” von der Leyen said.
To try and fill this gap, the Commission has also agreed with Pfizer/BioNTech that 50 million doses that were due in the third quarter of 2021 will now be delivered in Q2. This will bring the total doses delivered by Pfizer to 250 million doses in the second quarter. These will be distributed pro-rata to the population, among all the member states.
The US Centers for Disease Control (CDC) and the Food and Drug Administration (FDA) have called a temporary halt to the use of Johnson & Johnson’s COVID-19 vaccine while they conduct a review, following six reports of rare blood clots.
CDC said it will convene a meeting of its Advisory Committee on Immunization Practices (ACIP) on Wednesday to further review these cases and assess their potential significance, with FDA subsequently reviewing that analysis.
As of April 12, more than 6.8 million doses of the single shot Johnson & Johnson vaccine have been administered in the US.
The six blood clots are similar to those seen with AstraZeneca’s vaccine in Europe. All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination.
“Right now, these adverse events appear to be extremely rare. COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously,” the joint CDC/FDA statement said.
After concluding that very rare blood clotting events are linked to the AstraZeneca COVID-19 vaccine, the EMA safety committee has now started a review of reports of blood clots in people who have received the Johnson & Johnson vaccine.
Four serious cases of unusual blood clots with low blood platelets have been reported following vaccination with Johnson & Johnson’s product, one in a clinical trial and three cases occurring in the vaccine rollout in the US. One of them was fatal.
The Johnson & Johnson vaccine is currently only available in the US, but was authorised in the EU on 11 March 2021. Vaccine rollout has not started yet in any EU member state but is expected in the next few weeks.
EMA said the reports of blood clots – which have the same unusual presentation as those seen with the AstraZeneca vaccine - point to a “safety signal” but it is currently not clear whether there is a causal association.
The agency also announced its started a review of a safety signal to assess reports of capillary leak syndrome in people who were vaccinated the AstraZeneca vaccines. There have been five cases of the very rare disorder, which is characterised by leakage of fluid from blood vessels, causing tissue swelling and a drop in blood pressure.
The European Medicines Agency’s safety committee has concluded that unusual blood clots are a very rare side effect of AstraZeneca’s COVID-19 vaccine, but said the benefits of using it still far outweigh the risks.
It is not recommending any restrictions on who should receive the vaccine, saying there is no evidence of any specific risk factors, including any greater risk for women, or for younger age groups.
The safety committee carried out a review of 62 cases of blood clots in the brain and 24 cases of clots in the abdomen, reported in the EU drug safety database, as of 22 March 2021. Of these, 18 cases were fatal. Around 25 million people had received the AstraZeneca vaccine at this point.
There is now more data to review, with a total of 169 cases of brain clots and 53 cases of abdominal clots reported up to 4 April. Around 34 million people had been vaccinated in Europe (including the UK) by this date. EMA said the more recent data do not change the recommendations.
EMA is sending out a message to healthcare professionals and people receiving the vaccine to be aware of the possibility of blood clots combined with low levels of blood platelets occurring within 2 weeks of vaccination, setting down the symptoms and the particular features of the clots. The agency said the condition can be treated if the signs are recognised and appropriate drugs are given.
“The reported combination of blood clots and low blood platelets is very rare, and the overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects,” EMA said.
It will be up to member states to decide what action to take, depending on the pandemic situation and vaccine availability. Denmark, Netherlands and Latvia have stopped using the AstraZeneca vaccine, while others including France, Spain and Germany, are restricting its use to older people.
EMA has commissioned new studies and amendments to ongoing research to try and undercover the mechanisms behind the blood clots.
The UK’s Medicines and Healthcare products Agency stopped short of saying there is a causal link in its review of 79 cases of rare blood clots that have caused 19 deaths in the country. The link is stronger than seen previously, but not proven, and more research is needed, MHRA said.
MHRA is also updating its guidance to healthcare professionals to make them aware of the rare adverse events, but maintains the benefits are greater than the risks.
However, the Joint Committee on Vaccination and Immunisation (JVCI), which advises the UK government on the COVID-19 vaccines programme, said the risk/benefit profile for younger people has shifted as a result of the review. It changed its recommendations to say healthy people aged 18 – 29 should be offered an alternative to the AstraZeneca vaccine.
The French biotech company Valneva has announced positive data for the first part of the phase I/II clinical trial of its COVID-19 vaccine and said it plans to commence a phase III trial by the end of this month.
The phase I/II study, funded by the UK government, tested three different doses of the vaccine, which consists of whole, inactivated SARS-CoV-2 virus and an adjuvant to boost the immune response. Based on the data, Valneva has decided to advance the high dose into the phase III clinical trial.
The vaccine was generally safe and well tolerated across all doses, with no safety concerns identified by an independent safety monitoring board. More than 90% of all study participants developed significant levels of antibodies to the SARS-CoV-2 virus spike protein across all dose groups tested and the vaccine also induced broad T-cell responses.
The aim is to complete phase III and apply to the UK regulator, the Medicines and Healthcare products Regulatory Agency, for approval of the vaccine in the autumn 2021. Valneva said discussions with other regulatory bodies are ongoing.
Thomas Lingelbach, CEO of Valneva, said, “Given the potential advantages often associated with inactivated whole virus vaccines, we believe that [our vaccine] has an important role to play. This includes potential modifications to the vaccine to address variants, using our existing manufacturing process.”
In addition to funding the clinical trial, the UK government has invested money in Valneva’s manufacturing facility in Livingston, Scotland, where the inactivated virus is produced. The manufacturing process has already been upscaled to final industrial scale. From Scotland, the bulk material will go to the company’s plant in Solna, Sweden, where it will be put into vials.
In September 2020, Valneva struck a deal with the UK government to supply 60 million doses at a cost of €470 million. They were due to be delivered this year, but announcing the phase I/II results Valneva said the timeline for delivery will now extend into 2022.
Last month the UK government announced it would begin a revaccination campaign in the autumn, in an attempt to ensure protection from COVID-19 infection conferred by the current vaccination programme is maintained. Valneva said continues to work closely with the government to review plans, including meeting the UK’s booster campaign requirements, and on the potential development of a vaccine that is effective against emerging variants of the SARS-CoV-2 virus that threaten to undermine the effectiveness of existing vaccines.
Pfizer and BioNTech announced that their COVID-19 vaccine showed 100% efficacy and robust antibody responses in a phase III trial in adolescents 12 to 15 years of age. The results exceed those recorded earlier in participants aged 16 to 25 years old.
These are the topline results from a phase III trial involving 2,260 adolescents, who had not previously been infected with SARS-CoV-2.
“We share the urgency to expand the authorisation of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” said Albert Bourla, CEO of Pfizer. The data will be submitted to regulators in the coming weeks, “With the hope of starting to vaccinate this age group before the start of the next school year,” Bourla said.
The trial enrolled 2,260 adolescents 12 to 15 years of age in the US. There were 18 cases of COVID-19 in the placebo group and none in the vaccinated group. Vaccination elicited SARS-CoV-2–neutralizing antibody, demonstrating strong immunogenicity in a subset of adolescents one month after the second dose. This compares well with the response seen in participants aged 16 to 25 years old. The vaccine was well tolerated, with side effects generally consistent with those observed in participants 16 to 25 years of age.
The companies plan to submit the data to the FDA and EMA to request an amendment to the current approvals.
Last week, the first children were vaccinated in a global phase I/II/III seamless study to assess the safety, tolerability, and immunogenicity of the vaccine in children aged 6 months to 11 years of age.

 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.