This blog has been archived. A new one has been set up at this link.

The coronavirus pandemic is disrupting universities and research institutes across the world. But the same institutions are also working very hard to find out how the disease can be stopped and its effects mitigated.
Follow this live blog for the latest updates on how the crisis is impacting research and innovation, and what governments, funders, companies, universities, associations and scientists are doing to stop or cope with the pandemic.
You can read the full archive of this blog here.
The UK is offering its genomics resources to help countries that lack their own capabilities track variants of the SARS-CoV-2 virus that causes COVID-19.
The New Variant Assessment Platform is intended to provide an early warning of mutations that could increase the virulence of the virus, or reduce the efficacy of vaccines and therapeutics.
Countries will be able to access the platform via the World Health Organisation.
The UK has led the world in tracking the evolution of the SARS-CoV-2 genome in real time through sequencing the virus from COVID-19 patients. It currently sequences samples from 5% of patients, and has contributed half of the sequences in the global database.
The initiative will give an early warning of new variants of concern, said Isabel Oliver, director of Public Health England’s national infection service. “We know that the virus will evolve over time,” she said.
A key example is the B 1.1.7 variant, first identified in the UK in December, which is known to be more transmissible, and which initial data indicate is 30 – 40% more lethal. It has since been picked up in more than 60 countries. Other variants of concern have been identified in South Africa and Brazil.
The EU is threatening to introduce export controls for COVID-19 vaccines in response to a row with AstraZeneca over supplies of its vaccine, following reports that the company is unable to meet previous commitments on deliveries. That is said to be as a result of problems at one of the manufacturing facilities.
As a result, deliveries of the AstraZeneca/Oxford University COVID-19 vaccine to the EU are expected to be 60% less than promised during the first quarter of the year.
“In the future, all companies producing vaccines against COVID-19 in the EU will have to provide early notification whenever they want to export vaccines to third countries,” EU health commissioner Stella Kyriakides said on Monday.
The proposal follows frustration in Brussels over an agreement with AstraZeneca to deliver around 80 million of the 300 million doses the Commission has ordered by the end of March. According to the company, it now expects that amount to be cut to 31 million doses due to "production problems" at a manufacturing site in the European supply chain.
In a statement on Monday, Kyriakides said the company’s explanations for the delay “have not been satisfactory”. A call with AstraZeneca CEO Pascal Soriot was scheduled for Monday evening.
“This new schedule is not acceptable to the EU,” Kyriakides said. “We want clarity and full transparency. The EU has pre-refinanced the development and production of the vaccine and wants to see the return. The EU wants to know exactly which doses have been produced, where, by AstraZeneca so far, and if or to whom they have been delivered.”
On 8 December, the UK Vaccine Taskforce, set up to procure COVID-19 vaccines, said rather than domestic production, the initial doses of the AstraZeneca vaccine were to be imported into the UK from mainland Europe, to get the vaccination programme off the ground as soon as the vaccine was approved by the UK regulator. That happened on 30 December.
The vaccine is not yet approved by the European Medicines Agency, but its decision is expected before Friday.
Frustration with AstraZeneca is heightened by the EU's slow vaccine rollout. Cheaper and easier to distribute compared to the approved vaccines from Pfizer/BioNtech and Moderna, officials had hoped the AstraZeneca vaccine would give the European rollout a big push.
A COVID-19 vaccine based on technology originally developed by the Pasteur Institute in Paris and taken forward by Vienna-based Themis Bioscience, has been dropped by Merck & Co. Inc.
The US pharma company said the vaccine has failed to demonstrate good enough immune responses in phase I studies in healthy volunteers.
Merck said it is also dropping a second programme it was developing in collaboration with the non-profit organisation, AIDS Vaccine Initiative Inc (IAVI), to develop a COVID-19 vaccine based on the same technology as Merck used in its approved vaccine against Ebola virus.
According to the company, the immune responses to both vaccine candidates were inferior to those seen following natural infection and those reported for other COVID-19 vaccines.
This is a double blow, given Merck’s vaccines development and manufacturing capacity.
Merck announced it was acquiring Themis in May 2020, taking on development of the COVID-19 vaccine, which had previously received funding from CEPI, the Coalition for Epidemic Preparedness Initiative.
As the US re-engages with the World Health Organisation (WHO), it will also be joining WHO’s COVAX and ACT-Accelerator initiatives, set up to spur the development of vaccines and therapies, and to ensure their distribution in poorer countries.
“I am pleased to announce today that the US plans to work multilaterally to respond to and recover from the COVID-19 pandemic,” Anthony Fauci, the new US chief medical officer told a meeting of executive board of WHO this morning.
“President Biden will issue a directive later today which will include the intent of the US to join COVAX and support the ACT-Accelerator to advance multilateral efforts for COVID-19 vaccine, therapeutic, and diagnostic distribution, equitable access, and research and development,” Fauci said.
Fauci also thanked WHO for the way it has responded to the pandemic. “Under trying circumstances, this organisation has rallied the scientific and research and development community to accelerate vaccines, therapies and diagnostics; conducted regular, streamed press briefings that authoritatively track global developments; provided millions of vital supplies from lab reagents to protective gear to health care workers in dozens of countries; and relentlessly worked with nations in their fight against COVID-19,” he said.
The annual Altmetric Top 100, highlighting research and academic commentary published in 2020 that generated significant international online attention and discussion, found – unsurprisingly – that Covid-19 research accounted for roughly 30% of the top 100 papers.
These were spread across many disciplines including biomedicine, built environment and design, and economics.
Virus transmission and face mask protection were among the most heavily discussed topics in the analysis of 87.7 million mentions of 3.4 million papers.
The top articles on Covid-19 include:
-
Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers
-
Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1
-
It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19)?
-
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro
-
A modelling framework to assess the likely effectiveness of facemasks in combination with "lock-down" in managing the COVID-19 pandemic
A laboratory study to test if the Pfizer/BioNTech COVID-19 vaccine is capable of protecting against a variant of SARS-CoV-2 first detected in the UK, has found that blood samples from vaccinated people neutralise the virus.
The variant, called B.1.1.7, has ten mutations located in the spike protein against which most COVID-19 vaccines are designed. To test the impact on the effectiveness of their vaccine, researchers at Pfizer/BioNTech genetically engineered a replication-deficient virus with the full set of spike mutations. Sera from blood samples of 16 people who took part in a clinical trial of the vaccine inhibited this pseudovirus in a range said to be biologically equivalent to the effect on the original virus that emerged in Wuhan, China.
BioNTech and Pfizer previously published data from a study that evaluated N501Y, one of the key mutations in the B.1.1.7 variant, which is shared by the South African variant that is also causing concern. That study showed efficient neutralisation of the N501Y mutated virus by sera of individuals who had received the Pfizer/BioNTech COVID-19 vaccine.
The two companies said further studies are needed to monitor their vaccine’s effectiveness in preventing COVID-19 caused by new virus variants. It has not been established what reduction in neutralisation might indicate the need to modify a vaccine, but the underlying technology would enable such adjustment.
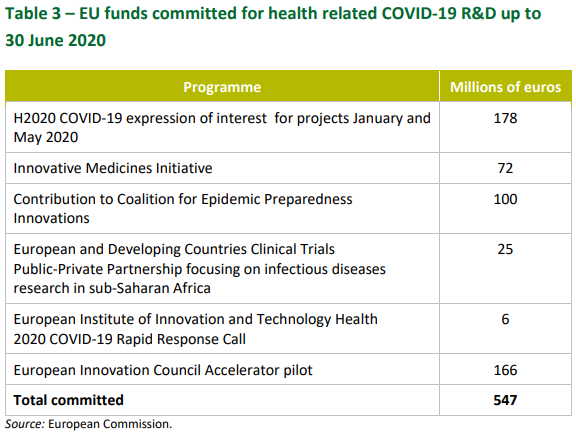
Health may be a member state competence and an area where the European Commission has very limited powers and influence, but a review of the EU’s initial contribution to the public health response by the European Court of Auditors shows it diverted €547 million to research on COVID-19 between January and June 2020.
The Commission first committed €10 million Horizon 2020 funding for research on COVID-19 on 31 January 2020, using the provision in its work programme that had already committed this amount in case of a public health emergency.
This funding was increased to €48 million by the end of March to support 18 projects (out of 89 eligible applications) developing vaccines, diagnostics, new treatments and monitoring systems.
The Commission then launched a further call for funding applications in May 2020, with a total budget of €130 million, to support 23 projects.
The Innovative Medicines Initiative, the public private partnership between the Commission and the pharmaceutical industry, also launched a call for funding applications in March. It selected eight projects focusing on diagnostics and treatment development, which will receive in total €117 million, €72 million of which is in grants from the European budget.
Then, in April, the Commission committed another €166 million of funding in April 2020 via the EU business accelerator programme, the European Innovation Council, to 72 companies working on innovative projects related to COVID-19.
The head of the World Health Organisation Tedros Adhanom Ghebreyesus, has hit out at the disparity in access to COVID-19 vaccines, saying, “It’s right that all governments want to prioritise vaccinating their own health workers and older people first. But it’s not right that younger, healthier adults in rich countries are vaccinated before health workers and older people in poorer countries.”
There will be enough vaccine for everyone, Ghebreyesus said. “But right now, we must work together as one global family to prioritise those most at risk of severe disease and death, in all countries.”
Over the past nine months, WHO has been working through the ACT Accelerator and the COVAX vaccines programme to lay the ground for the equitable distribution and deployment of vaccines. It has secured two billion doses of vaccines from five producers, with options on more than one billion more doses. The aim is to start deliveries in February.
But several WHO member states have questioned whether COVAX will get the vaccines it needs, and whether high income countries will keep the promises they have made to donate vaccines.
“The promise of equitable access is at serious risk,” Ghebreyesus said. More than 39 million doses of vaccine have now been administered in at least 49 higher income countries. Just 25 doses have been given in one lowest income country.
“I need to be blunt: the world is on the brink of a catastrophic moral failure,” said Ghebreyesus. “Even as they speak the language of equitable access, some countries and companies continue to prioritise bilateral deals, going around COVAX, driving up prices and attempting to jump to the front of the queue.”
The situation is compounded by the fact that most manufacturers have prioritised getting regulatory approval in rich countries where the profits are highest, rather than submitting full dossiers to WHO, according to Ghebreyesus. “It’s not too late. I call on all countries to work together in solidarity to ensure that within the first 100 days of this year, vaccination of health workers and older people is underway in all countries,” he said.
The European Medicines Agency said it has begun the rolling review of Johnson & Johnson’s single dose COVID-19 vaccine, weighing up the evidence that is available currently, in advance of a formal application for marketing approval from the company.
The European Commission has agreed to buy 200 million doses, with the option to secure up to 200 million additional doses.
Johnson & Johnson announced last month that it has completed recruitment to the phase III trial of the vaccine, enrolling approximately 45,000 participants. There is a high level of COVID-19 infection in the countries where the trial is being conducted and the company expects there will be enough cases to show if the vaccine is effective by the end of January.
If so, Johnson & Johnson said it expects to apply for emergency use approval from the US FDA in February, with applications to other regulators made in parallel.
The latest data from the phase I/IIa trial published this week in the New England Journal of Medicine, show the vaccine prompted an immune response that lasted for at least 71 days, in participants aged 18-55 years. After a single vaccination, neutralising antibodies against COVID-19 were detected in over 90% of study participants at day 29 and 100% of participants aged 18-55 years at day 57.
Data on durability of immune responses in trial participants aged over 65 years will be available in late January and longer-term follow-up to one year is planned.
The Swiss federal government has secured 7.5 million doses of Moderna’s COVID-19 vaccine following approval by the regulator Swissmedic, with first deliveries to the country expected to begin next week.
The authorisation is based on a rolling submission of data, including an analysis from the phase III clinical study published on 30 November.
The Moderna vaccine is also approved in the US, EU, Canada, Israel and the UK and is under review in other countries and by the World Health Organisation.

 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.