This blog has been archived. A new one has been set up at this link.

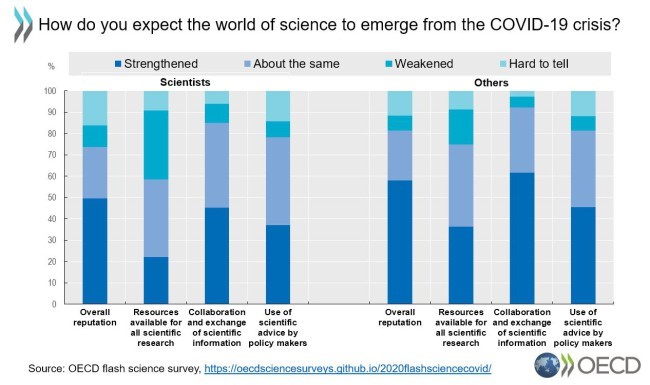
In an OECD flash survey, scientists, policy makers and the general public say they believe medical and scientific expertise will be more integrated in policy advice following the COVID-19 crisis. The findings, based on more than 1,100 responses from almost 80 countries, show that in addition to greater input to policy, respondents believe the reputation of science will grow further in the future, and scientific collaboration and exchange will be strengthened.
Zegami, a data visualisation start-up, has launched a scheme where scientists from around the world who are working on medical imaging projects can apply for a grant to use its software. Preference will be given to those working on Covid-19 projects. The Oxford University spin out has developed a proof of concept machine learning model, that uses X-rays of COVID19 infected lungs, artificial intelligence techniques and data visualisation tools that could help clinicians identify coronavirus cases more effectively, help provide a better idea of potential outcomes for patients, and even lead to more effective treatments. For the platform to reach its full potential, Zegami is trying to source a huge supply of COVID-19 X-rays and details on treatments used for patients and the outcomes.
As it puts the European supply chain in place for the commercial scale manufacture of its COVID-19 vaccine candidate AZD1222, Astrazeneca has contracted adenoviral vector specialist Cobra Biologics as a manufacturing partner for the vaccine, with first deliveries to begin in the UK in September 2020. The agreement builds on Cobra’s work as part of a consortium with the Jenner Institute at Oxford University, where the vaccine was designed, and others, to rapidly develop, scale-up and produce the recombinant adenovirus vector for the clinical trials.
The German Federal Minister for Economic Affairs and Energy, Peter Altmaier, and Dietmar Hopp, of the investment company dievini Hopp BioTech holding GmbH & Co. KG, announced the government will invest €300 million in CureVac AG, which is developing one of the lead COVID-19 vaccines. Altmaier said CureVac’s technology has the potential to develop disruptive new vaccines. “The German Federal government has decided to invest in this promising company because it expects that this will accelerate the development programmes and provide the means for CureVac to harness the full potential of its technology.
At the same time, today’s move is a first and important implementation of the comprehensive Corona Economic Stimulus and Future Technologies Package, which was agreed on by the Federal Government on 3 June 2020. With this package, we aim to secure more independence in the entire production process of medical substances and vaccines. This investment is a first step in this direction.” The government will hold a 23 per cent stake in CureVac.
Contract manufacturer Novasep has closed a deal with AstraZeneca to produce the viral delivery vectors for the COVID-19 vaccine originally developed at Oxford University. This follows the agreement between AstraZeneca and the Inclusive Alliance for Vaccines, whose members are France, Germany, Italy and the Netherlands, to supply the vaccine in Europe. Production will be carried out at Novasep's Belgian site in Seneffe.
The ‘Inclusive Vaccines Alliance’ of Germany, France, Italy and the Netherlands has struck a deal with AstraZeneca, giving access to the most advanced COVID-19 vaccine in development. Under the deal, the UK pharma company will supply 300 – 400 million doses of AZD1222, originally developed at Oxford University, with the first deliveries due before the end of 2020. Announcing the advance purchase agreement on Saturday (June 13), AstraZeneca CEO, Pascal Soriot thanked the Alliance for making it possible to supply Europe through a single deal, rather than having to negotiate separately with each government. “The four countries have done it on behalf of Europe,” Soriot said.
Last Friday, EU health ministers gave the European Commission a mandate to set up advance purchase agreements for the EU27, for up to six COVID-19 vaccines in development, using a €2.3 billion emergency fund. Speaking on Friday, health commissioner Stella Kyriakides said the Alliance would now “converge” with the commission’s procurement initiative.
If it is shown to be effective, Soriot said AstraZeneca will supply AZD1222 at cost to countries around the world, at an expected price of €2 per dose. Whether or not the vaccine protects against COVID-19 infection will be known by the end of August/early September. By that time, 50,000 volunteers will have taken part in trials, providing a safety database that Soriot said is as large as any vaccine ever developed before.
EU health ministers have given the commission the go ahead to start negotiating advance purchase agreements for COVID-19 vaccines on behalf of the EU27. “There is overwhelming political support” for initiative, said health commissioner Stella Kyriakides, speaking after a council meeting this morning. “Working together we will increase our chances to obtain a safe and effective vaccine, at the scale that we need, and as early as possible,” she said. The commission will now open a call for manufacturers, and is looking to make down payments on up to six vaccines, using the €2.3 billion remaining in the Emergency Support Instrument.
A study by researchers at Oxford University’s Institute for Global Health has found only 34 per cent of authors of COVID-19 journal papers are women, which they say suggests a significant gender bias in the research response to the pandemic. The study, which assessed 1,235 papers, shows women are especially under-represented in the most senior positions, making up only 29 per cent of first authors, who contribute most to the work, and 26 per cent of last authors, who are usually the project leaders. The researchers say the underrepresentation of women in COVID-19 research may result in an incomplete understanding of the sex and gender impacts of the pandemic, and that allowing voluntary disclosure of gender when submitting papers would help monitor gender inequalities and encourage teams to foster equality.
The European Investment Bank (EIB) today announced it will support global development and scale up of manufacturing by German vaccines developer BioNTech, through a €100 million debt financing agreement. The money, backed by two EIB programmes, will be handed out in two tranches of €50 million, following the completion of pre-defined milestones. BioNTech is testing four different mRNA vaccine candidates in phase I/II trials in the US and Europe which began in May. The company previously signed commercialisation agreements with US pharma Pfizer and Chinese pharma Fosun Pharmaceuticals, through which it received $250 million upfront, with the two partners responsible for most of the development costs.
Six member states have sent a letter to the European Commission calling for improved pandemic response measures. In research and innovation, they want to see bigger investments and a more coordinated EU approach, including joint vaccine development. The commission should examine whether EU should aim to scale up innovation capacity by strengthening Europe’s clinical trials network, and how EU-funds could be used to finance large scale clinical studies. The letter, initiated by Denmark and signed by Belgium, France, Germany, Spain, and Poland, also calls for more efficient data sharing and monitoring, better supply coordination, strengthening of the regulatory framework of the single market to ensure free flow of goods, and increasing production facilities within Europe, to reduce dependence on imports.

 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.