This blog has been archived. A new one has been set up at this link.

The Coronavirus Global Response pledging event coordinated by the European Commission has exceeded its original target of €7.5 billion to reach €9.8 billion. The pledging marathon that started on 4 May will now see the launch of a new campaign organised by with the international advocacy organisation Global Citizen, which will culminate in a Global Pledging Summit on Saturday 27 June. The European Commission will act as patron, with Bloomberg Philanthropies, the Bill & Melinda Gates Foundation and the Wellcome Trust as partners.
The Commission’s independent Economic and Societal Impact of Research and Innovation (ESIR) expert group is calling for research policies that put greater resilience by design at the core of a coordinated EU recovery response to the COVID-19 outbreak. In the midst of a global health emergency and with recession imminent, an integrated “people, planet and prosperity” recovery model, and concerted investment in research and innovation-led transformation, will enable the EU to emerge from this pandemic more resilient as a region, and as member states, ESIR said. The group posted a series of R&D recommendations for crisis and post-crisis management.
The European Commission has published the roadmap for its pharmaceutical strategy and opened it up for public comment, saying the current pandemic underlines the importance of building more resilient supply chains. In the commission’s view, the EU is too dependent on third countries for supplies of medicines and pharmaceutical ingredients. The industry body, the European Federation of Pharmaceutical Industries and Associations (EFPIA) disputes this, saying the experience of its member companies is that they have been able to increase supply to meet the needs of patients across Europe.
The industry says it already has a strong in-built resilience with 76 per cent of the active pharmaceutical ingredients used in the manufacture of patented drugs in Europe being sourced in the EU, and a further 11 per cent coming from the US. Only 9 per cent are sourced from Asia, including South Korea and Japan. The data underline the need for a nuanced, evidenced-based dialogue identifying all the root causes of drug shortages so that problems can be identified and addressed without jeopardising supply chains that designed to work across borders and over €400 billion in European exports of medicines, EFPIA says. The deadline for comments on the roadmap is 7 July.
A risk analysis carried out by a team of entomologists, virologists, and insect disease experts at Wageningen University & Research, the University of Groningen and the University of Copenhagen, has concluded there is a negligible risk of insects produced as food for humans or feed for livestock transmitting COVID-19. The SARS-CoV-2 virus that is responsible for COVID-19 invades human host cells by locking onto ACE2 receptors on the cell surface.
While insects also have ACE2 receptors, they are structurally so different from those in mammals that the virus cannot bind to them. Moreover, extensive analyses of the micro-organisms present in insects carried out in recent years have never recorded a virus from the wider group of coronaviruses to which SARS-CoV-2 belongs. In addition, the researchers note the production of insects as food and feed takes place in closed facilities that are subject to strict hygienic measures and the processes in the facilities are highly automated. As a result, contact of humans with the insects is negligible, thus strongly reducing the chance of passive transmission.
Two researchers at the San Diego Supercomputer Center have been awarded a $200,000 National Science Foundation grant to fund the collection of diverse sources of COVID-19 information into a transdisciplinary knowledge network that integrates health, pathogen, and environmental data, to better track cases and to improve analysis and forecasting. The award is one of 13 funded under the NSF’s Rapid Response Research programme, which focuses on research that can be used immediately to explore how to model and understand the spread of COVID-19, to inform and educate about the science of virus transmission and prevention.
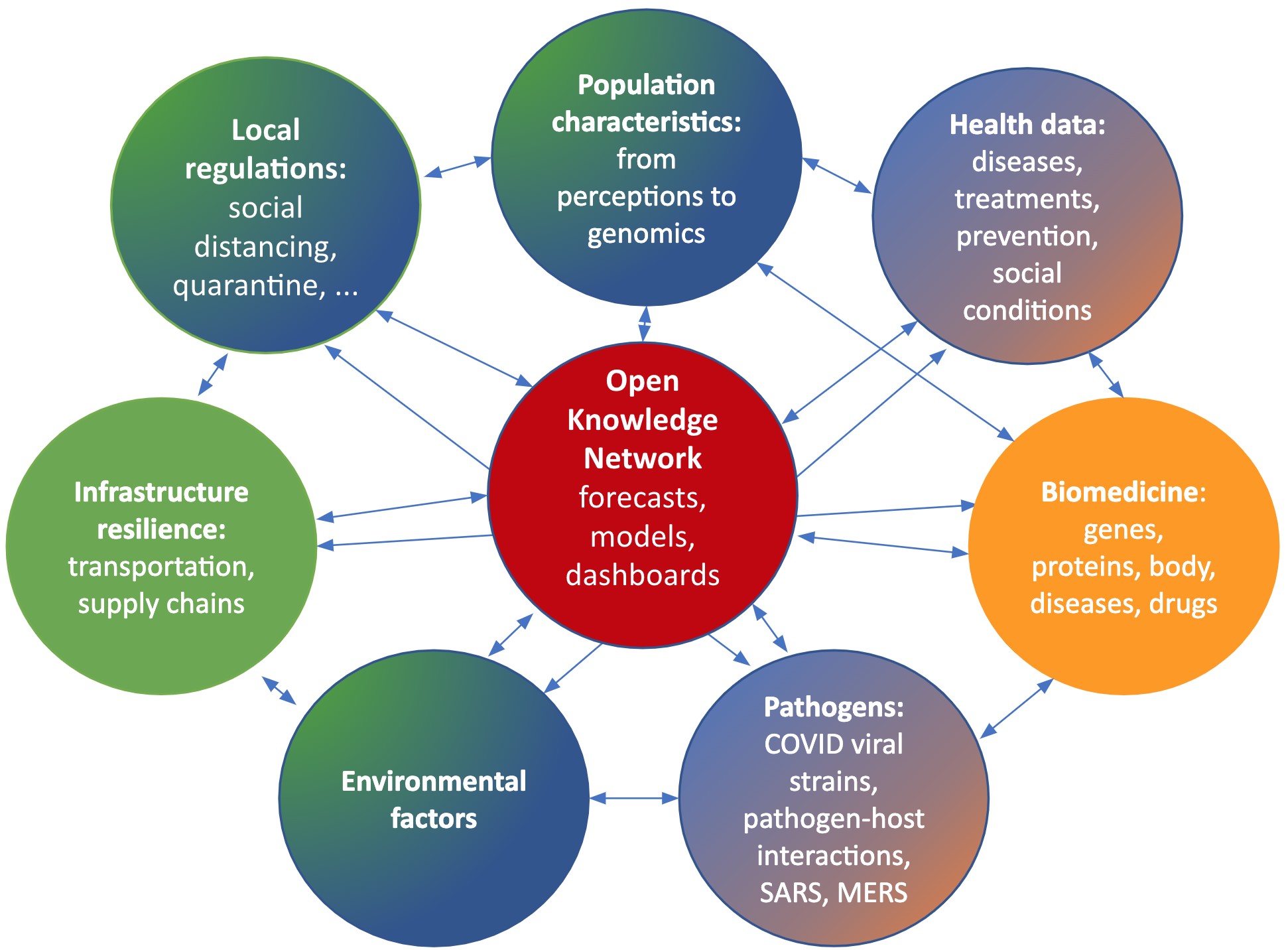
Complementary knowledge graph themes to be integrated into a comprehensive Open Knowledge Network (OKN) by project partners: UC San Diego (blue), UC Santa Barbara (green), and UC San Francisco (orange). Gradients represent areas of overlapping expertise and integration. Credit: Krzysztof Janowicz (UC Santa Barbara), Peter Rose and Ilya Zaslavsky (San Diego Supercomputer Center, UC San Diego)
The Austrian Research Promotion Agency has selected 45 projects to be funded through the country’s €26 million COVID-19 research fund, with the last 21 awardees announced on Saturday. Across the country, 18 research projects will work on COVID-19 diagnostics, 16 will look for ways to prevent the spread of the disease and nine will search for new drugs. The grantees include two Vienna-based vaccine developers, Themis Bioscience, which last week announced it is being acquired by the US pharma company Merck, and Baxalta Innovations, owned by Japanese pharma company Takeda.
Vinnova, Sweden’s public research funding agency, today announced two new COVID-19 research opportunities for projects looking to curb the spread of the disease and prevent future pandemics. Successful proposals in the control of COVID-19 will receive up to SEK 5 million (€500,000), while feasibility studies to help prepare for the next pandemic are eligible for up to SEK 1 million (€100,000). Only private or public Swedish organisations can lead projects, but foreign organisations eligible to receive funding in Sweden are welcome as partners. Both calls are open until 18 August 2020.
The Australian government is investing an additional AUS$ 60 million (€40 million) in its Medical Research Future Fund, adding to AUS$ 30 million already pledged for the coronavirus research response. The funding will be used for the discovery of vaccines and antiviral therapies, for clinical trials and to improve the responsiveness of the healthcare system.
BerGenBio of Bergen, Norway, said dosing has commenced in the UK government-funded phase II clinical trial of its cancer drug bemcentinib, in hospitalised COVID-19 patients. Bemcentinib was selected as the first candidate to be tested as part of the Accelerating COVID-19 research and development study, launched on 28th April 2020 to rapidly test potential drugs in early stage clinical trials and if positive, feed them into the UK's large-scale phase III COVID-19 Recovery trial, which to date has recruited 11,080 patients at 176 hospitals around the country.
The first patient was treated at Southampton University Hospital and the study is open in a further seven sites across the UK. It will recruit 120 subjects in total, assessing the safety and efficacy of bemcentinib as an add‑on to standard of care in 60 patients, with a control group of 60 patients receiving standard or care alone.
Bemcitinib is in development as a treatment for a number of cancers. The drug is an inhibitor of Axl kinase, an enzyme cancer cells express to evade the immune system. The decision to test it in COVID-19 is based on preclinical data showing the drug is also a potent inhibitor of the coronavirus in several cell types.
Researchers at the Crick Institute in London and Charité – Universitätsmedizin Berlin have identified 27 protein biomarkers that could be used to predict whether a patient with COVID-19 is likely to become severely ill with the disease. Some people infected with SARS-CoV-2 do not develop any symptoms, some need to be hospitalised and, for some, the disease is fatal. The 27 potential biomarkers are present in different levels in patients with COVID-19, depending on the severity of their symptoms. In addition to helping to predict how ill a patient will become, these could provide new targets for drug discovery.
The researchers used mass spectrometry to rapidly test for the presence and quantity of various proteins in blood plasma of 31 COVID-19 patients at the Berlin University hospital Charité. Their results were further validated in 17 patients with COVID-19 at the same hospital, and in 15 healthy people.

 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.