Tiny sensor within pill signals smartphones when it reaches gut, providing a record for patients and doctors
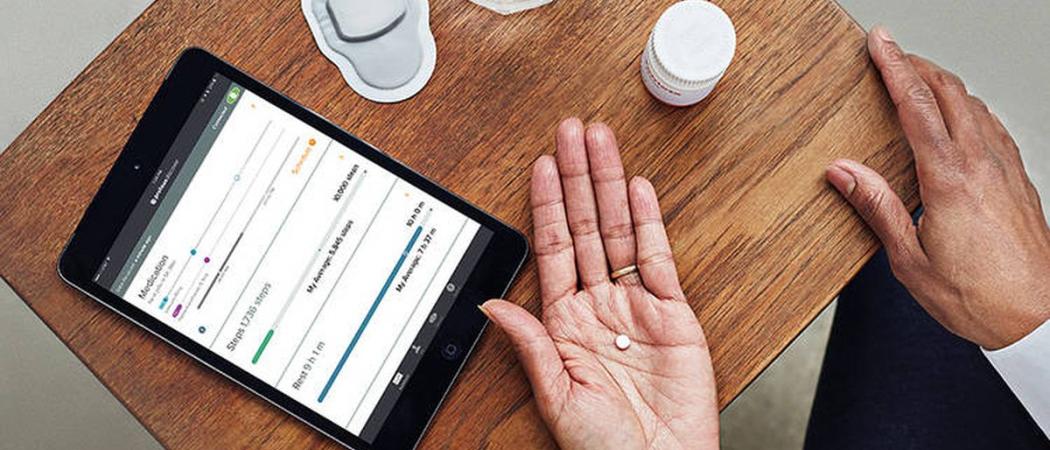
Photo: Proteus Digital Health
The US Food and Drug Administration has approved a first-of-its-kind digital drug which signals a smartphone after it has been ingested.
The drug, Abilify MyCite, dissolves when it comes into contact with stomach acid, exposing a sensor the size of a grain of sand, which communicates wirelessly with a wearable patch.
The patch then relays the information to a smart phone app, which also collects data on heart rate, body position and activity levels.
The pharmaceutical element of the drug-device combination is aripiprazole, which is approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar disorder, and for use as an add-on treatment for depression in adults.
The sensor comes from Proteus Health Systems, a Silicon Valley company, and aripiprazole from the Japanese pharma Otsuka. The price of the drug-device combination is yet to be agreed.
Patients can indicate through the app who can access to their information. Doctors should ensure patients are capable of and dedicated to using the system before prescribing it, says the FDA.
Analysts say that tracking devices within pills could save the healthcare system, and patients themselves, a lot of money. Failing to stick to a regular medication schedule is a common problem for millions of patients, leading to other problems that require more serious treatment further down the line.
But so far, there is no evidence that the new pill improves adherence. The FDA warns that the digital pill should not be used to track drug ingestion “in real-time” or during an emergency, because detection may be missed or delayed.





 A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.
A unique international forum for public research organisations and companies to connect their external engagement with strategic interests around their R&D system.